|
|
|
| HOME > Research > Research Field |
| |
| Research Field |
| |
 |
| |
 Energy efficient designs Energy efficient designs |
| |
 Intensification of reaction and separation Intensification of reaction and separation |
|
|
The intensification of reaction and separation proposes dramatic economic savings, a higher production yield, and a circumvention of pinch points. Dramatic economic savings come from the simplification of a complex process and the utilization of a reaction heat for a distillation. The shift in chemical equilibrium resulting from the simultaneous separation and removal of the products cause a higher production yield. Also this shift of chemical equilibrium leads to the circumvention of pinch points. The main task in realizing this technology is to visualize the interaction between reactions and separations on the basis of reaction and phase equilibriums.
|
|
|
| |
 Feasibility of a continuous reactive distillation Feasibility of a continuous reactive distillation |
|
|
The most important question when intensifying reaction and separation is whether the combination of reaction and distillation can be achieved at a desired reaction. It can be answered on the basis of analyzing the visualized composition space. From understanding of reaction, phase equilibriums, a quick and reliable algorithm is proposed to evaluate the feasibility of various kinds of reactive distillations. From this proposed method, the feasibility of a reactive distillation for a desired reaction can be checked without numerous experiments and simulations.
|
| |
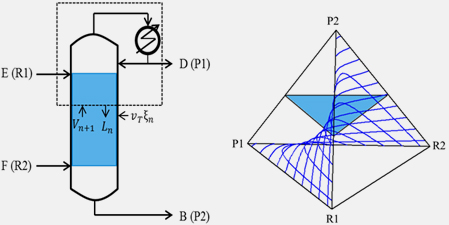 |
Figure 1. A basic schematic of a reactive distillation and tetrahedral composition space of reaction and phase
equilibriums |
|
|
| |
 Feasibility of a batch reactive and batch reactive extractive distillation Feasibility of a batch reactive and batch reactive extractive distillation |
|
|
A batch distillation has been used for small-scale production such as pharmaceuticals and noble chemicals. A feasibility evaluation method is also proposed for the batch distillation on the basis of analyzing residual curve maps. By classifying various reactions on the positions of singular points in the residual curve map, a desired reaction can be evaluated whether it produce pure products through a batch distillation.
When the intensification of reaction and separation for a desired reaction is not feasible, pinch points can be circumvented by introducing the proper entrainer. The reason of this circumvention is also proved from visualized composition space.
|
| |
 |
| Figure 2. Basic schematics of batch distillations |
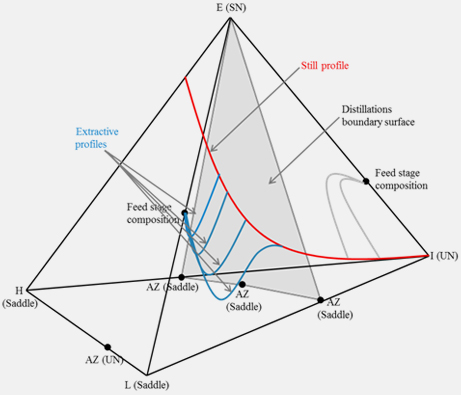 |
| Figure 3. Circumvention of pinch points in batch extractive distillation |
|
|
|
|
| |
|